CAPA Software
The concept of Corrective and Preventive Actions, required by GMP and numerous ISO/FDA regulations such as ISO 13485 and FDA 21 CFR 820, is a key method of quality management in the medical device industry. With a cross-departmental software solution such as CAQ.Net you can not only carry out CAPAs effectively, but also have a globally applicable tool at your disposal with which you can monitor, control, and document the entire quality system. CAQ.Net provides various techniques for different facets of quality management, which it combines in one modular software.
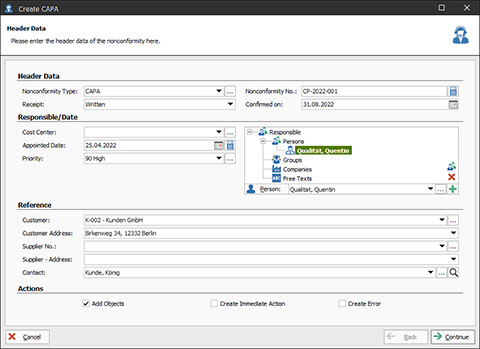 CAPA-Assistant in the CAQ.Net Software
CAPA-Assistant in the CAQ.Net Software
Corrective and Preventive Actions
With the CAPA concept, you can identify possible causes for quality defects that have occurred, then solve them and adjust workflows, products, or manufacturing processes to prevent the defect from recurring. In this context, the entire production process, from supplier selection and incoming goods inspection to quality inspection and complaints processing, should be visible and necessary data should be available at all times.
Why CAPA Software?
However, if the integrity of received goods or quality inspection and complaint management processes are documented manually or maintained in separate spreadsheets, two key capabilities to run an effective CAPA system are already missing: information transparency and data compatibility. The CAPA Software CAQ.Net is used in order to facilitate these capabilities and maintain a complete PDCA cycle.
CAPA in CAQ.Net
CAQ.Net software consists of all components that are necessary to monitor and document the quality of products – throughout the entire product life cycle from the product’s initial creation to subsequent complaint management processes that may arise. Thus, in the event of a CAPA, you can access all quality-related data of a product with just one system and identify the true causes of a quality defect by means of comprehensive root cause analysis. This means that you can use just one system to determine whether the root cause of the quality defect, for instance, lies in the purchased raw material, a defective production step, or inadequately trained personnel.
Assistant-Supported CAPA Software
Since the reason for triggering a CAPA can occur in many different areas of quality management, the CAPA process in CAQ.Net is not implemented as a stand-alone software module, but is available as a cross-modular, assistant-supported function throughout the entire system. If, for example, the trigger of a CAPA is identified in gauge management, training management, audit management, or complaint management, you can initiate the CAPA process directly in the corresponding CAQ.Net module by following a few steps in the assistant.
Avoiding CAPAs with CAPA Software
CAQ.Net is a globally applicable tool with which the entire quality system can be monitored, controlled, and documented so that the reasons for a possible CAPA can be eliminated as best as possible in advance. This is important not least because, contrary to widespread opinion, the CAPA process in the sense of the FDA is rather the last resort in quality management and should not be initiated excessively.